Acidosis in beef cattle under intensive feeding systems : causes and prevention
Pr. Sergio Calsamiglia, from the Universitat Autònoma de Barcelona, Animal Nutrition & Welfare Service.
Intensive Beef Cattle Farming Systems
High concentrate diets used in intensive farming can result in acidosis, impacting negatively animal welfare, production and profitability.
The use of sodium bicarbonate is the best preventive strategy, helping in controlling acidosis and bringing economic advantages.
How does an intensive beef production system work?
Intensive production systems achieve high beef growth rates
The need to feed an ever-increasing global population has led to the growth of intensive farming due to its ability to produce large amounts of food in a small area of land at a lower unit price. By using an intensive farming system for beef cattle production, it is possible to achieve high beef growth rates in relatively short cycles, with an average daily gain ranging from 1.0 to 2.0 kg/d and a conversion index of 4 to 6.5 kg of feed per kg of live weight gain, depending on age and breed.
High concentrate diets are more acidogenic
This rate of growth is achieved using high concentrate diets which are more energetic but also more acidogenic. Acidosis is a critical nutritional disease for beef cattle since it can negatively affect intake and digestibility of the ration, reduce growth and feed efficiency and increase the incidence of bloat, inflammation and foot problems.
All these factors can lead to a reduction in animal production and profitability, but also in animal welfare.
Getting the right ruminal pH balance
In order to be effective, cattle ration formulations need to seek a balance between the use of carbohydrate levels to maximize energy intake and the normal-optimal pH of the rumen, which should remain in the range of 6.2 to 7.0.
Ruminal pH depends on 3 factors
- The production of acid, mainly linked to the rate of starch degradation
- The buffer capacity of the ruminal environment, with saliva secretion representing the main factor
- The elimination of protons by absorption or flow to the lower digestive tract.
Acidosis episodes are often the result of a de-synchronization between acid production and buffering capacity.
The important role of buffers against acidosis
Sub-acute ruminal acidosis can be controlled with the use of buffers.
A buffer is simply a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid and it works by reacting with any added acid or base to control the pH.
The use of sodium bicarbonate
The most commonly used buffer in intensive fattening ruminant diets is sodium bicarbonate. The recommended levels of bicarbonate in feedlot cattle were studied by a group (Gonzalez et al., 2008a, 2008b) using diets formulated with 0, 1.25, 2.5, and 5.0 % bicarbonate in the concentrate.
The results showed that cattle fed with 1.25% sodium bicarbonate
- had a better and more homogenous distribution of the daily intake in several small meals throughout the day
- spent more time ruminating
- had the lowest number of hours of pH below 5.8.
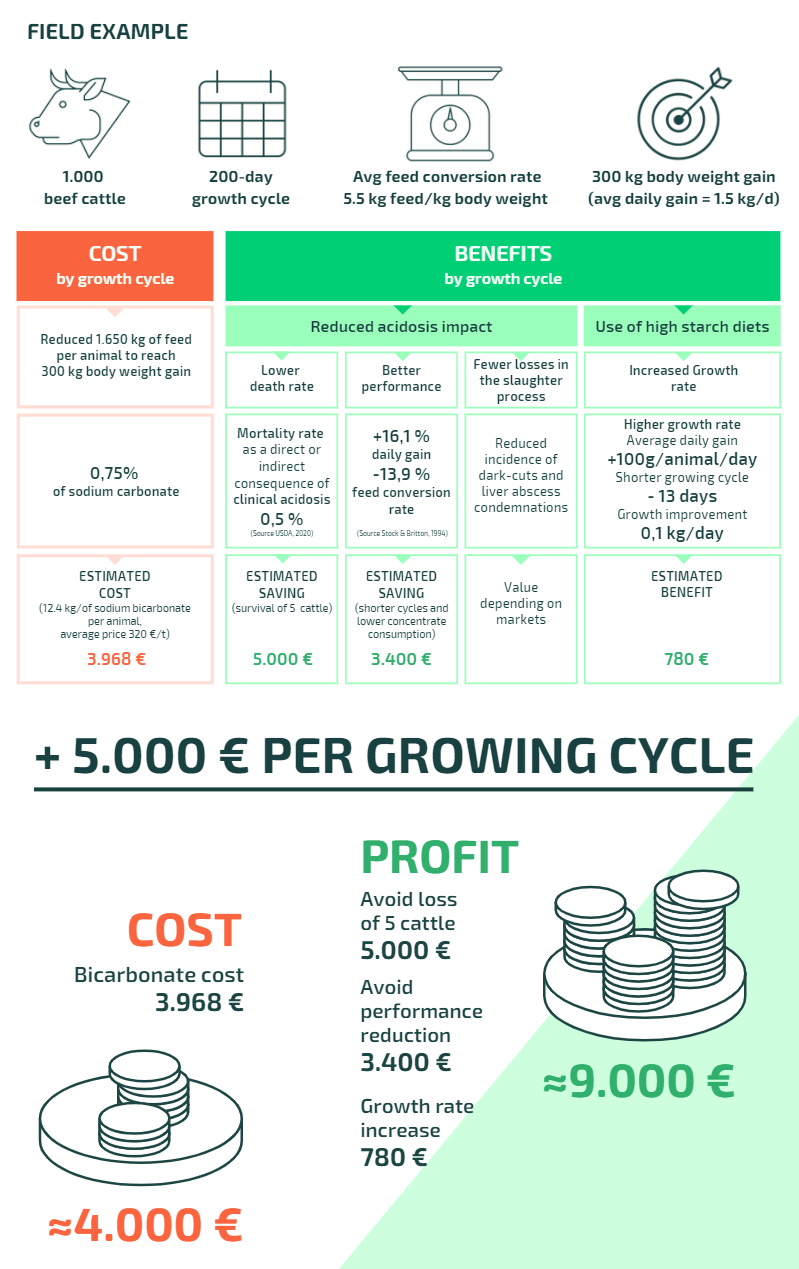
Weighing the economic benefits of using sodium bicarbonate
It is important to identify the main causes of acidosis in beef cattle under intensive feeding systems and define the best preventive strategies. However, while the use of sodium bicarbonate has been shown to bring advantages to animal welfare, what are the economic advantages for farmers?
Conclusion
The cost-benefit analysis demonstrates the economic return of using sodium bicarbonate as a preventative measure against acidosis in beef cattle.
The result of using sodium bicarbonate as a preventative measure is more than a two-fold gain in profit and clearly points to the all-round advantage of maintaining a stable ruminal pH.
To achieve this objective, it is necessary to carefully formulate the levels of fiber and non-fibrous carbohydrates and add a sodium bicarbonate buffer between 0.75 to 1.25% of the dry matter.